by Raashid Andrabi
SRINAGAR: America’s Food and Drug Administration (FDA) has finally approved the Kashmir cardiologist’s life-saving device that helps in managing pulmonary embolism. The device has been named after its inventor Dr Riyaz Bashir as Bashir Endovascular Catheter and will be now in use in the USA and abroad.
Dr Riyaz Bashir is a Srinagar-born cardiologist who works at the Temple University Hospital.
“Riyaz Bashir was frustrated with the tools he had to treat blood clots in the lungs,” The Philadelphia Inquirer reported. “So the Temple University Hospital cardiologist invented a new device to treat the nation’s third-leading cardiovascular cause of death.”
The FA approval came in April 2023. “Hopefully it will benefit a lot of patients, not just in this country but around the globe,” Bashir was quoted as saying. The device was developed through a collaboration between Temple and Thrombolex Inc., a Bucks County company that will develop and sell it. Bashir is the co-founder of the company that will make and sell the device.
The device will find its use in removing the blood clots that get stuck in one of the arteries in the lung. This makes blood inaccessible to oxygen as a result of which deaths take place.
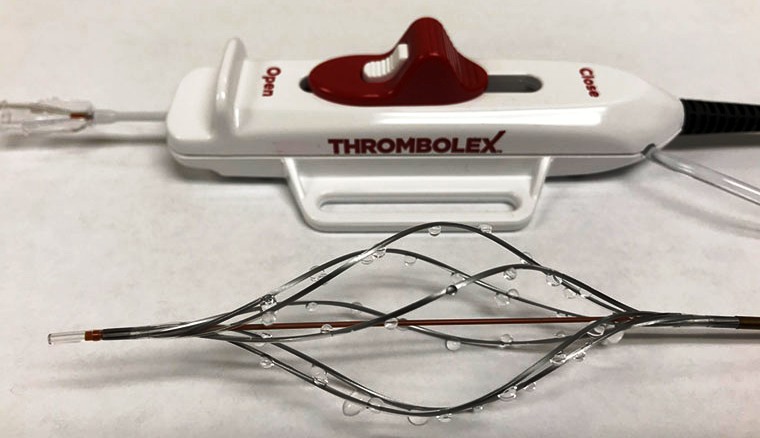
Though similar catheters exit for the heart and legs but unclogging the comparatively larger arteries in the lungs with these devices was challenging and difficult. Now Bashir’s invention has emerged as a brand new device for managing the particular artery set-up.
“Bashir’s invention, involving a flexible tube called a catheter, looks like the medical equivalent of a children’s spin toy,” the newspaper reported, detailing the possible 15-minute procedure using the new device. “A cardiologist inserts the device through a vein in the groin or neck and guides it to the clogged artery in the lung. Then it penetrates the blockage. Six small tubes with holes ring the catheter. Once in the clot, these tubes spin, creating a tunnel that allows blood to pass through.”

The approval came after trial runs. Bashir and his co-authors made the findings public in December 2022 in a paper published by the Journal of the American College of Cardiology: Cardiovascular Interventions, in which it showed the “amazing” results from 109 patients in which the device was used for treatment. The device removed blockages faster with less clot-dissolving medicine and the rate of bleeding was low.
From an idea to a device, the invention took a long time. It took a lot of funds too. Dr Bashir told Kashmir Life Science Talks that the biggest source of funding for the initiative was made by the federal government and the Commonwealth of Pennsylvania. “They have provided the US $5 million so far for the development of the device,” he said. “But as a whole, it took the US $17 million dollars for the device to reach the stage where FDA approved it for use.”
Bashir Catheters is actually is a basket of seven devices of which five are designed for the legs and two are designed for the lungs.
The Temple cardiologist said that when the idea was initially shared with the medical engineers, there was less positivity. “Ultimately one of the engineers agreed to design the tool because he was himself suffering from such type of blood condition and had also lost a friend to it,” Bashir said. “So, with all the engineering and creative processes, it took us a year to finally produce the prototype of the tool.”