by Dr Muzafar Jan
For the third time in as many decades, a zoonotic coronavirus (CoV) has crossed species to infect human populations. Until the 2000s, CoVs were considered minor pathogenic for humans, generally associated with a common cold or mild respiratory infections in immunocompetent people, with rare exceptions in infants, young children and elder people. For example, there are already four well-described CoVs which are low pathogenicity coronaviruses endemic in humans: HCoV-OC43, HCoV-HKU1, HCoV-NL63 and HCoV-229E.

This concept and perception about CoVs completely changed with the emergence of highly pathogenic zoonotic disease, Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS)caused by SARS-CoV and MERS-CoV, respectively. SARS (November 2002 to July 2003) was a coronavirus that originated from Beijing, China, spread to 29 countries, and resulted in 8,096 people infected with 774 deaths (fatality rate of 9.6%). Considering that SARS ended up infecting 5,237 people in mainland China, Wuhan coronavirus surpassed SARS on January 29, 2020, when Chinese officials confirmed 5,974 cases of the novel coronavirus (2019-nCoV). One day later, on January 30, 2020, the novel coronavirus cases surpassed even 8,096 cases worldwide which were the final SARS count in 2003. MERS (in 2012) killed 858 people out of the 2,494 infected fatality rates of 34.4%). MERS-CoV was suggested to originate from bats but the reservoir host fuelling spillover to humans is unequivocally dromedary camels.
It is interesting to mention that SARS-CoV and MERS-CoV are closely related to each other and originated in bats which most likely serve as a reservoir host for these two viruses. Whereas palm civets and racoon dogs have been recognized as an intermediate host for zoonotic transmission of SARS-CoV between bats and humans. With the revolution in next-generation sequencing technology, a bloom of viruses were discovered, uncovering thousands of virus sequences in wild animals population around the world. The recurrent spillovers of coronaviruses in humans along with detection of numerous coronaviruses in bats, including many SARS-related coronaviruses (SARSr-CoVs), suggest that such zoonotic transmission events were already well speculated by scientists but not addressed thoroughly for some unknown reasons remains a question to be answered. A clear realization of the possibility of breaking species barrier, lack of therapeutics or vaccines against any of infecting coronaviruses gives room to a lot of ambiguous concerns raised by the researchers around the world.
A previously unknown coronavirus, named SARS-CoV-2, was discovered in December 2019 in Wuhan, Hubei Province of China and was sequenced and isolated by January 2020. The origin of the outbreak was linked to Huanan Seafood Wholesale Market. Clinical signs, similar to SARS and MERS, are fever, cough and difficulties in breathing, with evolution to severe pneumonia in the most severe cases. After a few days from the outbreak announcement, Chinese scientists obtained and published genomic sequences of the putative aetiological agent, a novel β-coronavirus, provisionally called 2019-nCoV eventually classified as SARS-CoV (SARS-CoV-2). SARS-CoV-2 is associated with an ongoing outbreak of atypical pneumonia (Covid-2019) that has as of 18 March 2020 (15:00hrs) infected 199,325 people with 7,994 deaths and 82,795 recoveries worldwide. Among current 108,536 active cases, there were 102,118 (94%) infected patients with mild symptoms/condition and 6,418 (6%) were serious or critical. Among currently 90,789 closed cases with an outcome, 82,795 (91%) were either recovered / or discharged and 7,994 (9%) had dies. Till date, the virus has affected 163 countries. The countries most affected with CoV include China, Italy, Iran, Spain, Germany, South Korea and France. At the time of writing this article, India has 152 cases.

The characteristic that makes this virus as a global public threat is its highly contagious transmission route. For a non-science knowing person, the human-to-human transmission process for this virus is exactly similar to common cold or flu. We in Kashmir are especially aware of how we catch a common cold at home, workplaces, public transport etc. Although, as per WHO (world health organization) report every year an estimated 290,000 to 650,000 people die in the world due to complications from seasonal influenza (flu) virus, there are other reasons that make this virus a significant threat to mankind. The high level of mortality associated with seasonal flu which as per WHO corresponds to 795 to 1781 deaths per day exceeds many folds compared to the current mortality rate of COVID19. COVID-19 posses a challenge to scientists to address:
- We don’t understand zoonosis or how virus breaks species barrier and spills to into human from wild: What made the SARS-CoV-2 jump or cross-species barrier (Zoonosis) from bats to humans? It has been known for three decades now that CoVs have great zoonotic potential. The high diversity between CoVs detected in bats and the genetic mechanisms to increase their genomic variability increases the risk of interspecies transmission. The emergence of the SARS-CoV-2 highlights the importance of bats as a reservoir for new viruses capable of infecting humans. But we currently lag sophisticated design and strategies to develop viral models that can provide knowledge to prevent new dangerous spillover events.
- The mutation potential of the virus is very high leading it into more virulent/and drug-resistant forms: SARS-CoV-2 is a plus-strand RNA virus and the main difficulty with these viruses is that they are highly mutable under selection pressure. When the virus infects the human body, it copies its genetic material to progenies to produce and mature new viruses within its host. The copying of genome is carried out by enzymes which are usually highly precise. But in case of SARS-CoV-2 and other RNA viruses, there is no proofreading mechanism for these enzymes so they are born to accumulate mutations. Within the host, the virus is always under selection pressure imposed by the reactive host immune system. In order to thwart the host immune system, the virus mutates and more often results in more virulent forms. Similarly, in the presence of antiviral drugs targeted at viral protease, and RNA polymerase etc. they mutate and form drug-resistant viruses which are not then killed by these anti-viral drugs. Currently, we do not have studies available which have addressed these issues but from the preliminary investigations, the virus shows evolutionary potential to more virulent forms as well as the tendency to form drug-resistant forms. I am not sure whether the virus displays the same level of transmission as the original one in China. These mentioned factors are highly discouraging for the general perspectives pertaining to this pandemic, its rate of transmission and containment of the pandemic especially under circumstances when no workable vaccine is available against.
- The viral spike is immunologically silent and developing a vaccine against is tough: Coronavirus entry into human cells is mediated by the transmembrane spike (S) glycoprotein, the spike which gives the appearance of the corona to the virus under the microscope and from which the name of the whole family Coronaviridae, subfamily Coronovirinae is derived. This spike is a mini-robot which holds the entire information and machinery to identify the host cells (for example human lung cells), then starts a landing process via holding the receptor on host cells using its spike which eventually culminates into the fusion of virus and cell and finally unloading its genetic material (viral RNA) into the host cell. As a result, coronavirus entry into susceptible cells is a complex process that requires the concerted action of receptor-binding and proteolytic processing of the S protein to promote virus-cell fusion. Different coronaviruses use distinct domains within the spike to recognize a variety of attachment and entry receptors, depending on the viral species. Endemic human coronaviruses OC43 and HKU1 attach via their S domain A (SA) to 5-N-acetyl-9 O-acetyl-sialosides found on glycoproteins and glycolipids at the host cell surface to enable entry into susceptible cells. MERS-CoV Suses spike to engage non-acetylated sialosides as attachment receptors and promote subsequent binding to the entry receptor, dipeptidyl-peptidase 4. SARS-CoV and several SARS-related coronaviruses (SARSr-CoV) interact directly with angiotensin-converting enzyme 2 (ACE2) via spike to enter target cells.
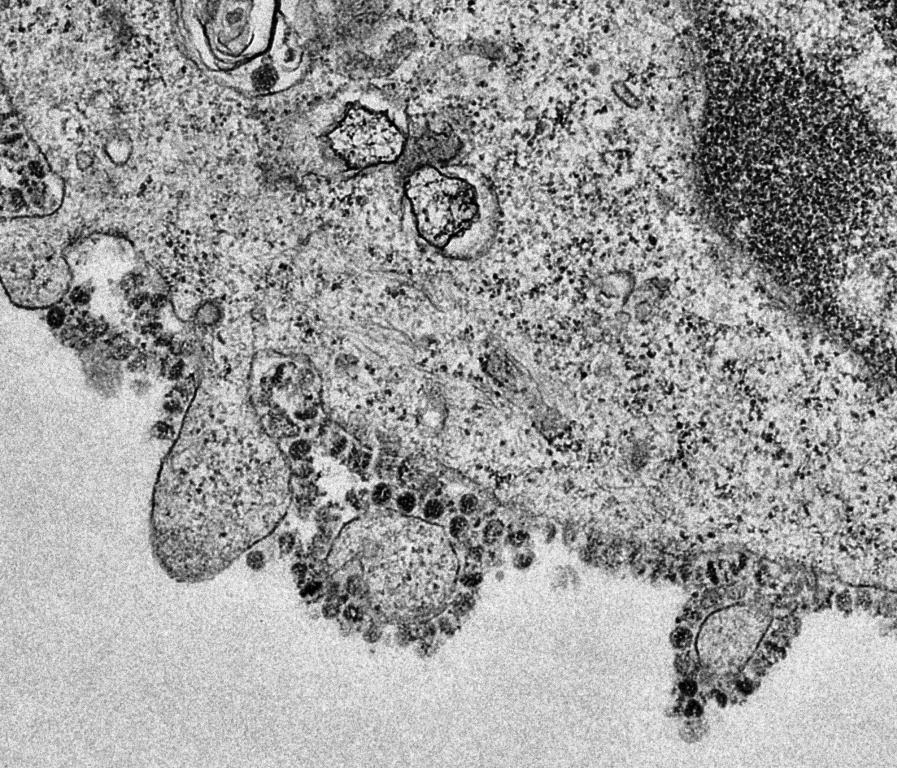
The spike on SARS-CoV-2 is the only entity which is foreign to the host immune system and has immunogenic properties. Normally our immune system has characteristic that it can recognise only foreign substances entering a body like microbes, viruses etc. The CoVlife cycle includes a strategy for the virus to grows and generates itself within the human host cells (lung cells). The virus that is generated from the infected human cell derives its lipid bilayer, covering the exterior surface of the virus, from the host cell rendering it immunologically silent (our immune system cannot detect the virus made of our own body). The spike being central to viral infectivity and pathogenicity is recognised by the human system as foreign (because it is encoded by the viral genes not human)and protective response is generated against this only viral protein presented to the immune system. However, the virus has an escape strategy even against this immune recognition by densely coating its spikes with carbohydrates again of host origin. Carbohydrates being omnipresent in our body are not normally recognised by the immune system. This escape strategy of the virus to coat its only immune target with carbohydrates does not allow our immune system to generate antibodies against the virus. We are all aware that effective vaccines need to develop antibodies in our body against the antigen. For example, we get vaccines early in childhood against polio, pertussis, diphtheria etc. only to have life long antibodies to prevent these fatal infectious diseases. So, the possibility of developing antibodies under these viral (SARS-CoV-2) escape strategies by our(host) might be scarce at large, except in very few fortunate individuals whose immune system may explore some novel targets on spike protein and develop antibodies against the virus and protection from the disease. This viral escape strategy and lack of immune response is another impediment to tackle the virus and development of a vaccine against SARS-CoV-2.
It appears that the virus is becoming more transmissible between humans. It should be closely monitored whether the virus continues to evolve to become a more virulent, more pathogenic or drug-resistant form. A proper vaccine against SARS-CoV-2 can be developed only by thoroughly investigating and studying those patients who have already recovered from this disease. Those patients must have developed some novel strategies to fight this virus and probably may have developed protective antibodies against the virus. Studying these few protected individuals and their immune response particularly antibodies may give us a gross understanding of what are the stable targets of those antibodies in the spike of SARS-CoV2. So, we have to again learn from nature how it deals with this virus to combat it in its host.
Considering the widespread of SARSr-CoV in their natural reservoirs, future research should be focused on active surveillance of these viruses for broader geographical regions. In the long term, broad-spectrum antiviral drugs and vaccines should be prepared for emerging infectious diseases that are caused by this cluster of viruses in the future.
Most importantly, strict regulations against the domestication and consumption of wildlife should be implemented.
(The author is Voccinologist and Virologist, having Ph D, Post Doctoral Fellow, (Virology Unit) New York School of Medicine, NYC, USA & Icahn School of Medicine at Mount Sinai, NYC, USA. He is currently associated with the latter institution.)